Participating in PREMIUM
Please note recruitment is closed, thank you for your interest.
Who can participate in this study?
- Female or male with overweight/obesity;
- Healthy;
- Between 18 and 50 years of age;
- With no food restrictions;
- Not pregnant or breastfeeding;
- Non-smoker.
What will happen in the study?
Participants will attend at least 4 clinic visits over a 14-week period. The day and time of your visits will be decided by you and the study coordinator. We will first collect a blood sample to determine if you are eligible for the study. If eligible, you will be randomly assigned to a powdered meal replacement group or control group. Participants in the powdered meal replacement group will add the meal replacement to their diets twice daily for 12 weeks. Participants in the control group will maintain their usual food intake. The meal replacement is neutral tasting powder which you will add to water and drink. The study coordinator will show you how to do this. Besides from taking the meal replacement, no other lifestyle changes are needed and maintain your normal medication regime and physical activity level is required. You must inform study staff if you make any changes to your current medication or nutritional supplement use. You cannot participate in the study if you take any natural health products which may alter inflammation, gut microbiome, energy metabolism, body weight and composition, or hormone levels. You should also continue to eat your normal diet. You will weigh yourself daily during the study using a scale we will give you. It is important that you do not lose or gain weight during the study. If this happens you will meet with a registered dietitian to adjust your food intake. Over 12 weeks we will collect three blood samples to measure the level of inflammation, different hormones, genes related to nutrient metabolism and gene expression, fat, and sugar in your blood. We will also collect three stool samples to study the microbes living in your gut and three urine samples to study the substances you consumed with the meal replacement (only if you are in the powdered meal replacement group). You will also complete different questionnaires that ask you about your food intake and level of physical activity. Moreover, for the powdered meal replacement group, you will answer questionnaires about how hungry or full you feel.
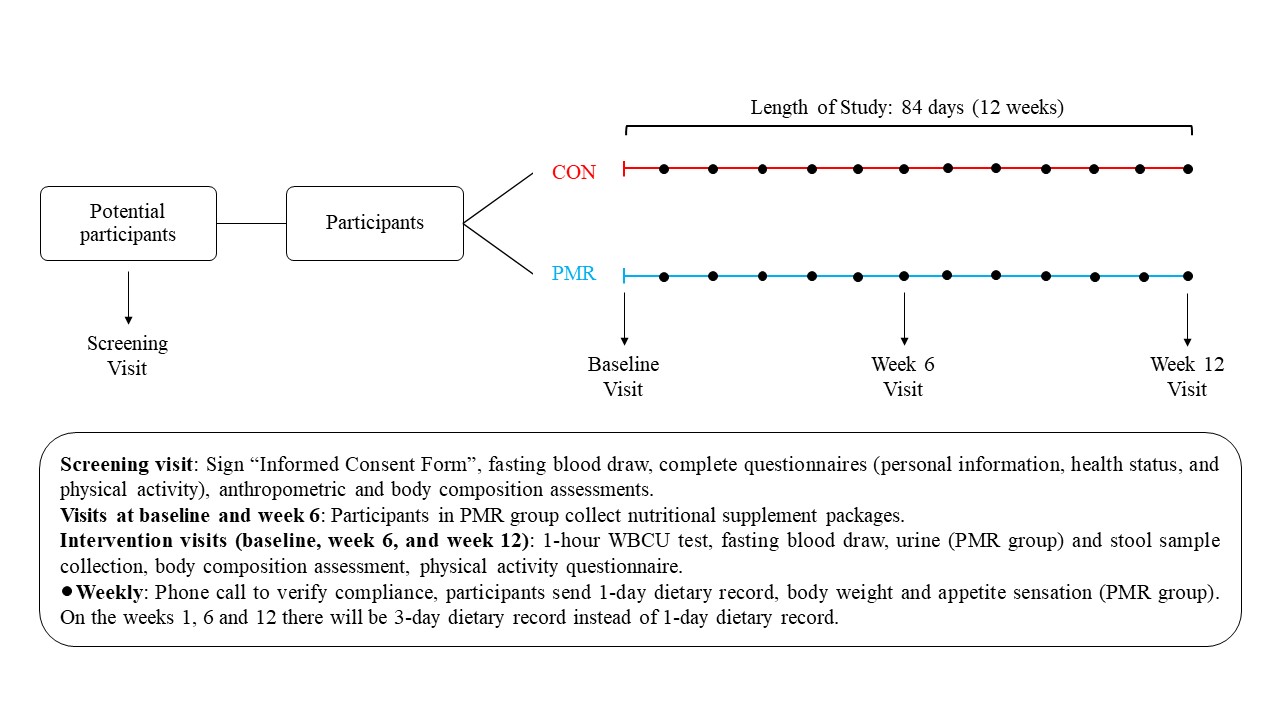
Screening Visit (about 1 hour):
This visit will happen in the morning. For this visit you should not consume alcohol for 3 days and not eat or drink for 12 hours before the visit. You may drink water. First, the study coordinator will explain the study to you. If you are interested in participating, you will be asked to sign this consent form. Then trained personnel will collect 10 mL of your blood (2 teaspoons). This blood sample will help us determine if you are eligible to participate based on your liver, kidney and thyroid function, and hydration status. If one or more of these tests is outside the reference values for a healthy person, you will be notified and considered not eligible to participate in our study. You may not participate in this study if you are pregnant, the blood test will tell us if you are currently pregnant. During the study, those who are of childbearing potential, must practice adequate methods of birth control (e.g., total abstinence, hormonal birth control methods (oral, injectable, transdermal, or intra-vaginal), intrauterine devices, confirmed successful vasectomy of partner etc.). If you become pregnant over the duration of the study, you must stop taking the product, and immediately inform the study investigators. You will also answer questions related to personal characteristics, health status and physical activity. We will measure your height, weight, and waist circumference. We will also measure the fat in your body using bioelectrical impedance analysis (BIA). You will lie down on a bed and eight self-adhesive electrodes will be connected to both of your hands and feet. You will not feel anything, and the procedure will take 1 to 2 minutes. Or you will be asked to stand on a scale with the ball and the heel of each foot in contact with four metal electrodes. This measurement will take no more than 15 seconds. At the end of this visit we will offer to you a snack and a beverage. Once we test your blood, we will contact you to let you know if you are eligible. If so, you will be randomly assigned to a powdered meal replacement group or control group and we will schedule your second visit.
Baseline Visit (about 2 hours):
This visit will also happen in the morning. For this visit, you should not consume alcohol for 3 days and not eat or drink for 12 hours before the visit. You may drink water. You should not exercise 24 hours before this visit and do as little activity as possible before the visit. For example, taking the elevator instead of the stairs.
At the beginning of this visit we will estimate how many calories you burn during a day using the whole-body calorimetry unit (WBCU). The WBCU is similar to a small hotel room. It has a bed, armchair, table, sink, toilet, television, computer/internet and treadmill. You will lie in a relaxed position on a bed. You will breathe regularly and relax without falling asleep. The test will take about 1 hour.
Then, we will measure the fat and muscle in your body using 3 safe and routine techniques. The first technique is a dual energy X-ray absorptiometry [DXA] scan, which may happen in a different day than the visit but in the same week or as close as possible. Before the scan we will make sure it is safe for you by asking some questions. If you are a woman, we will test your blood sample to see if you are pregnant. Pregnant women will not be allowed to participate in this study. During the scan you will lie down on a bed and the technician will position you correctly. The equipment “arm” will pass over your body and it will not cause any discomfort to you. It will take about 20 minutes to finish this test. The second technique is air displacement plethysmography (ADP). During this test you will sit comfortably inside a chamber and stay relaxed and quiet for 1 minute. This will happen twice. The entire test will take about 5 minutes. For this test, you will need to wear minimal, form-fitting clothing and a swim-cap. The last technique is the BIA, you will lie down on a bed and eight electrodes will be connected to both of your hands and feet. You will not feel anything, and the procedure will take 1 to 2 minutes.
After these assessments, certified personnel will collect 24 mL of your blood (5 teaspoons) to assess your level of inflammation, hormones, the interaction between your genes and the diet, fat, and sugar in your blood. If you are in the powdered meal replacement group, you will also collect your urine (~1 cup) to assess the level of substances contained in the meal replacement.
Then, we will give you a snack to eat. You will then answer a physical activity questionnaire. The study coordinator will explain how to monitor your body weight and complete questionnaires for the study. These questionnaires will ask about how hungry and full you feel and the foods you eat.
We will also explain how to collect a stool sample at home using the kit provided. You can bring this stool sample back later the same day or the next day. Once we receive your first stool sample, the research coordinator will give to participants assigned to the powdered meal replacement group the packages with the product and explain how to use it. The product will be mixed with water and taken twice daily as snacks in the morning and afternoon for 12 weeks (84 days). Lastly, the study coordinator will provide you with another stool collection kit.
Week 6 Visit – Day 42 (about 2 hours):
This visit will happen in the morning and is similar to the baseline visit, except genetic analyses, that will not be assessed. This means that the amount of blood we will collect will be 20 mL (4 teaspoons). For this visit, you should not consume alcohol for 3 days and not eat or drink for 12 hours before the visit. You may drink water. You should not exercise 24 hours before this visit and do as little activity as possible before the visit. During this visit we will complete the same assessments as Visit 2. You will also bring your stool sample to this visit. If you are unable to bring it to this visit, you can bring on the day before or after your scheduled appointment.
The study coordinator will check if your weight has changed from the beginning of the study and review your study journal. If your weight has changed the dietitian will work with you to adjust your diet to make sure your weight remains stable. At the end of the visit, the study coordinator will provide you with another stool collection kit. Participants in the powdered meal replacement group should keep taking the meal replacement mixed with water twice daily as snacks in the morning and afternoon until Visit 4. Participants in the control group will maintain their usual food intake.
Week 12 Visit – Day 84 (about 2 hours):
For this visit you will prepare as you did for baseline visit. This will be the last visit you will attend. For this visit you should bring back the scale provided during the study period and your final stool sample. As with the other stool sample collections, you are able to bring the sample to the visit, or the day before or after the scheduled visit. The study will have been completed at the end of this visit.
Weekly Assessment / Communication:
At the beginning of the study, we will provide a study journal. This provides reminders of what needs to be done every day throughout the study. Daily and weekly tasks include weighing yourself and completing questionnaires. These questionnaires will be about how hungry and full you feel (only for participants assigned to the powdered meal replacement group) and the foods you eat. Before Visits 2, 3, and 4 you will complete an online questionnaire about the food you ate three days during the weeks of these study visits. This will include one weekend day and one weekday. For the other weeks of the study, you will complete one of these questionnaires each week. The study coordinator will call or email you each week. This is to make sure you are following the recommendations provided. You will also be asked about your daily weight measurements and reminded to complete your study journal.
Results:
You will learn how many calories you burn in a day. You will also receive information about the amount of fat, bone, and lean soft tissue (i.e., everything else, but fat and bone) in your body.
What are the risks and discomforts?
There are no known risks of eating the meal replacement, there may be unknown risk with taking this investigational natural health product and potential side effects may include liver related drug adverse events. It is very unlikely to cause you any discomfort. The blood draws are a routine procedure performed by trained personnel. A needle will be inserted into a vein and blood will be withdrawn for lab tests. It is possible you may experience mild pain, fainting, bleeding, and bruising, and or an infection at the insertion site. Bruising is common, but usually goes away after a few days. Infection, dizziness, and fainting are rare during this procedure. There are no risks of having the genetic analysis, only potential general genetic linkages with metabolism of nutrients will be identified. You may also feel uncomfortable being alone in the WBCU. However, the tests will take only 1 hour and there will always be research staff close by and there is an intercom system to talk to them.
The X-ray dose associated with DXA scan is very low and not believed to have any long-term bad effects on your health. Pregnant women are excluded as a precaution. Having a DXA scan does not make it unsafe for you to have other X-rays in the future.
The BIA test is a risk for you only if you have a pacemaker or other internal electrical medical device. This is due to the risk of device malfunction from the weak electrical signal. Individuals with pacemakers or internal medical devices will not be able to participate in this study.
Dr. Laurie Mereu is a member of our research team and medical doctor. She will review your blood tests. If there are abnormal results Dr. Mereu will provide suggestions on how to proceed.
What are the benefits to me?
There are no direct benefits to you for participating in this study. We hope the study will give us more information about how our bodies use the powdered meal replacement.
What happens if I am injured because of this research?
If you become ill or injured as a result of being in this study, you will still be able to receive necessary medical treatment. This will occur at no additional cost to you. By signing this consent form, you are not releasing the investigators, institution, or sponsors from their legal and professional duties.
Do I have to participate?
No. Taking part in this study is your choice. You may stop participating in the study at any time. You can withdraw by contacting a study coordinator. Phone number: (780) 492-7820.
Will I be paid to be in the research?
After you complete the study, we will compensate your time with a $300 honorarium. We will also give to you a parking pass in case you need to park your car in front of our clinic. There is no cost associated with participating.
Will my information be kept private?
During the study we will collect your health information. This will be kept private. We will not release information containing your name outside of the study investigators office. It will not be listed in the research when published. By law, we may have to release your information with your name so we cannot guarantee absolute privacy. However, we will make every legal effort to make sure that your health information is kept private.
During research studies it is important that the data we get is accurate. For this reason, your health data and name may be looked at by people from the University of Alberta auditors or members of the Research Ethics Board. By signing this consent form, you are giving permission for the study staff to collect your health information and use it for research purposes.
After the study is done, we will securely store your health data that was collected as part of the study. As per Health Canada requirements, your data will be stored and kept confidential for 25 years. If you leave the study, we will ask permission to keep your data. If you do not respond, we will use your data that had been collected so far.
If you leave the study, we will not collect any new information from you. However, we will keep the data that we have already collected, unless you specifically request it to be destroyed.
What if I have questions?
If you have any questions about this research, please contact the study coordinator (Julia Montenegro at 780-492-4182).
If you suffer a research related injury, please contact the study coordinator at this number as well.
If you have any questions about your rights as a research participant, you may contact the Health Research Ethics Board at 780-492-2615. This office is independent of the study investigators.
